Pfiesteria shumwayae: Difference between revisions
imported>Candice Flemming m (→References) |
mNo edit summary |
||
| (62 intermediate revisions by 4 users not shown) | |||
| Line 1: | Line 1: | ||
{{subpages}} | {{subpages}} | ||
{{Taxobox | {{Taxobox | ||
| color = | | color = Turquoise | ||
| name = ''Pfiesteria shumwayae'' | | name = ''Pfiesteria shumwayae'' | ||
| image = P._shum.jpg | | image = P._shum.jpg | ||
| Line 15: | Line 14: | ||
| binomial_authority = | | binomial_authority = | ||
}} | }} | ||
==Description and significance== | |||
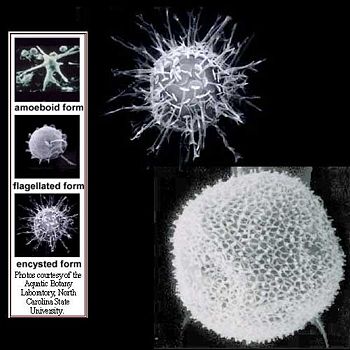
''Pfiesteria shumwayae'' is a heterotrophic dinoflagellate belonging to the protist group pyrrophycophyta. It was discovered by Dr. Burkholder at North Carolina State University in 1995. These dinoflagellates are found in warm coastal regions and temperate or tropical estuaries, and are known to cause massive fish kills via micropredation. It was once thought that fish kills in these areas were caused by exotoxin secretion by ''Pfiesteria'', however recent research has shown that the parasitic feeding behavior of ''P. shumwayae'' is responsible for denuding fish of their epidermis and causing characteristic lesions on dead fish.<ref name=Vogelbein2002>{{Citation | title = Pfiesteria shumwayae kills fish by micropredation not exotoxin secretion | url = http://www.bom.hik.se/bio710/artiklar/Vogelbein.pdf | year = 1984 | journal = Biotechnol. Bioeng. Symp | pages = 563–571 | volume = 14 | last1 = Vogelbein | first1 = W.K. | last2 = Lovko | first2 = V.J. | last3 = Shields | first3 = J.D. | last4 = Reece | first4 = K.S. | last5 = Mason | first5 = P.L. | last6 = Haas | first6 = L.W. | last7 = Walker | first7 = C.C. | accessdate = 2009-04-24}}</ref> They also prey on other protists, shellfish tissue, and micro-algal species such as cryptomonads[http://tolweb.org/Cryptomonads/2396]. ''P. shumwayae'' cysts and excysted offspring feed on the epidermis of live fish and free floating cells that detach from fish. ''Pfiesteria'' is known to exhibit both sexual and asexual reproduction, but asexual reproduction is most common among these dinoflagellates. <ref name=Parrow2003>Parrow, M.W. and Burkholder, J.M. 2003. Reproduction and sexuality in P''fiesteria shumwayae'' (Dinophyceae). J. Phycol. 39:697-711.</ref> | |||
It is also known that ''P. shumwayae'' can have a serious, negative effect on human health. Fisherman who are frequently exposed to ''Pfiesteria'' reported having respiratory irritation, rashes, sores and lesions on their skin. Researchers working with ''P. shumwayae'' have noted experiencing headaches, dizziness and short-term memory loss. However, there is no evidence supporting illness in individuals who consumed ''Pfiesteria'' exposed seafood <ref>North Carolina Public Health. "Pfiesteria." 2006. North Carolina Department of Health and Public Services. 23 Apr. 2008. <http://www.epi.state.nc.us/epi/oee/pfie.html</ref> | |||
It is also known that ''P. shumwayae'' can | |||
==Genome structure== | ==Genome structure== | ||
Not much research has been done to sequence the ''Pfiesteria'' genome | Not much research has been done to sequence the ''Pfiesteria'' genome in spite of the fact that researchers are mainly concerned with its harmful environmental effects. Much focus has been put on the actual mechanisms that attribute to fish kills and how to mitigate ''P. shumwayae'' damage to fish populations. | ||
==Cell structure and Feeding Habits== | ==Cell structure and Feeding Habits== | ||
''P. shumwayae'' is a biflagellate phagotroph that feeds with a peduncle, or feeding tube which extends from its spherical body. The transverse flagellum is wrapped around it and the other is free flowing. The peduncle is used to ingest epidermal cytoplasm and other food matter via myzocytosis | ''P. shumwayae'' is a biflagellate phagotroph that feeds with a peduncle, or feeding tube which extends from its spherical body. The transverse flagellum is wrapped around it and the other is free flowing. The peduncle is used to ingest epidermal cytoplasm and other food matter via [[myzocytosis]].<ref name=Vogelbein2002/> Direct dinospore attachment to the epidermal surface of fish create abrasions and damage to fish tissue such as gills, olfactory organs, oral mucosa and the lateral line canal. After completion of dinospore feeding, the peduncle is stretched thin and retracts as it detaches from the fish to swim away. <ref name=Parrow2003/> | ||
{{Image|deadfish.jpg|right|350px|Lesions on fish caused by ''P. shumwayae''}} | {{Image|deadfish.jpg|right|350px|Lesions on fish caused by ''P. shumwayae''}} | ||
Vogelbein ''et al.'' (2002), showed that cell organelles such as mitochondria and rough endoplasmic reticulum was observed as it passed through the peduncle during feeding. As fish cytoplasm is taken up by the peduncle, the dinospore increases in size because the ingested materials are transported to a food vacuole that occupies most of the cell. Staining of the dinoflagellates after feeding revealed that the food vacuoles within them were filled with nucleic acids and lipids. <ref name=Skelton2008>Skelton, H.M., Burkholder, J.M. and Parrow, M.W. 2008. Axenic Cultivation of the Heterotrophic Dinoflagellate ''Pfiesteria shumwayae'' and Observations on Feeding Behavior. J. Phycol. 44:1614-1624</ref> | |||
As ''P. shumwayae'' feeds, it uses its peduncle to pierce the cell membrane of its host, allowing host cell cytoplasm to flow through a small circular opening which contracts | As ''P. shumwayae'' feeds, it uses its peduncle to pierce the cell membrane of its host, allowing host cell cytoplasm to flow through a small circular opening which contracts.<ref name=Skelton2008>Skelton, H.M., Burkholder, J.M. and Parrow, M.W. 2008. Axenic Cultivation of the Heterotrophic Dinoflagellate ''Pfiesteria shumwayae'' and Observations on Feeding Behavior. J. Phycol. 44:1614-1624</ref> This sphincter, observed by Calado and Moestrup (1997), contracts in order to control the amount of cytoplasm being ingested into and pushed out of the dinoflagellate’s food vacuole. Calado and Moestrup suggested a theory of two different mechanisms in which suction serves as the driving force behind cytoplasm ingestion. | ||
During feeding, ''P.shumwayae'' exhibits cytoplasmic extensions or pseudopodia that are constantly extended and retracted for movement and attachment to host (Lom and Lawler, 1973). Up to six extensions were observed per cell. As the cell moves in response to chemosensory attraction, cytoplasmic extensions are left behind. Other flagellate cells are attracted by these entrails and were observed as they attached themselves to the entrails and ingested them | During feeding, ''P.shumwayae'' exhibits cytoplasmic extensions or pseudopodia that are constantly extended and retracted for movement and attachment to host (Lom and Lawler, 1973). Up to six extensions were observed per cell. As the cell moves in response to chemosensory attraction, cytoplasmic extensions are left behind. Other flagellate cells are attracted by these entrails and were observed as they attached themselves to the entrails and ingested them. <ref name=Skelton2008>Skelton, H.M., Burkholder, J.M. and Parrow, M.W. 2008. Axenic Cultivation of the Heterotrophic Dinoflagellate ''Pfiesteria shumwayae'' and Observations on Feeding Behavior. J. Phycol. 44:1614-1624</ref> After feeding on micro-algae and other prey, ''P. shumwayae'' leaves behind only the cell membrane; however it is noted that when it feeds on fish cells under axenic[http://www.thefreedictionary.com/axenic] laboratory conditions, no visible cell membrane is left behind. <ref name=Skelton2008>Skelton, H.M., Burkholder, J.M. and Parrow, M.W. 2008. Axenic Cultivation of the Heterotrophic Dinoflagellate ''Pfiesteria shumwayae'' and Observations on Feeding Behavior. J. Phycol. 44:1614-1624</ref> In addition, research documented by Schnepf and Deichgraber (1984) supports that the cell membrane is not ingested during or after ''P. shumwayae'' feeding. | ||
It has been noted that ''P. shumwayae'' can feed on prey several times larger than its own size; however feeding occurs on organisms that are usually injured ciliates, rotifers and nematodes. It has also been reported that the dinoflagellates can | It has been noted that ''P. shumwayae'' can feed on prey several times larger than its own size; however feeding occurs on organisms that are usually injured ciliates, rotifers and nematodes. It has also been reported that the dinoflagellates can ingest heterotrophic bacteria via phagocytosis, but this is an area that is in need of more research. <ref name=Skelton2008>Skelton, H.M., Burkholder, J.M. and Parrow, M.W. 2008. Axenic Cultivation of the Heterotrophic Dinoflagellate ''Pfiesteria shumwayae'' and Observations on Feeding Behavior. J. Phycol. 44:1614-1624</ref> | ||
==Toxicity== | ==Toxicity== | ||
The ''Pfiesteria'' species contains ''P. shumwayae'' and ''P. piscicida'', both of which have benign and toxic strains. The classification of toxicity in strains of ''Pfiesteria'' has to do with whether or not their exotoxins are inducible. In benign strains (NON-IND), toxins are not inducible; dinoflagellates are either not capable of toxic activity or the toxins they produce are negligible. Toxic strains that are actively toxic (TOX-A) or temporarily toxic (TOX-B) depend on whether there | The ''Pfiesteria'' species contains ''P. shumwayae'' and ''P. piscicida'', both of which have benign and toxic strains. The classification of toxicity in strains of ''Pfiesteria'' has to do with whether or not their exotoxins are inducible. In benign strains (NON-IND), toxins are not inducible; dinoflagellates are either not capable of toxic activity or the toxins they produce are negligible. Toxic strains that are actively toxic (TOX-A) or temporarily toxic (TOX-B) depend on whether there are live fish present.<ref name=Glasgow2001>{{Citation | ||
| title = … toxic Pfiesteria complex species and a conservative analysis of their role in estuarine fish kills | |||
| url = http://ehpnet1.niehs.nih.gov/members/2001/suppl-5/715-730glasgow/glasgow-full.html | |||
| year = 2001 | |||
| journal = Environmental Health Perspectives | |||
| pages = 715–730 | |||
| last1 = Glasgow | first1 = H.B. | |||
| last2 = Burkholder | first2 = J.A.M. | |||
| last3 = Mallin | first3 = M.A. | |||
| last4 = Deamer-melia | first4 = N.J. | |||
| last5 = Reed | first5 = R.E. | |||
| accessdate = 2009-04-24 }}</ref> In the presence of live fish, ''P. shumwayae'' exhibits immediate chemotaxis to fish and gradually becomes toxic. According to research done by Glasgow ''et al.'' (2001), it was demonstrated that shortly after fish death, TOX-A strains of ''Pfiesteria'' ceased production of toxins and transformed into an amoeboid form that fed on dead fish remains. In the presence of other micro-algal prey, ''P. shumwayae'' reverted to the less toxic TOX-B form. Optimal temperature for toxicity is greater than or equal to 25°C. | |||
{{Image|stages.jpg|left|350px|Life cycle stages}} | {{Image|stages.jpg|left|350px|Life cycle stages}} | ||
Experiments were done that indicate toxic strains of ''P. shumwayae'' use exotoxins to weaken fish. They can also have effects on mammals as well. Toxins secreted by dinospores are similar to an ATP transmitter that targets the P2X7 receptor | Experiments were done that indicate toxic strains of ''P. shumwayae'' use exotoxins to weaken fish. They can also have effects on mammals as well. Toxins secreted by dinospores are similar to an ATP transmitter that targets the P2X7 receptor <ref name=Glasgow2001/>, causing a severe inflammatory response. Cultured fish exposed to ''Pfiesteria'' toxins showed signs of depression, loss of equilibrium, hyper-excitability and decreased respiration. The physical, epidermal damage done to fish by ''Pfiesteria'' is speculated to increase the efficiency of toxins into fish tissues. Some strains of ''Pfiesteria'' that are less toxic do not kill fish unless it has direct contact with its prey.<ref name=Glasgow2001/> | ||
==Reproduction== | ==Reproduction== | ||
''P. shumwayae'' shares phylogenic relationships with and shows similar reproductive patterns to the marine diatom ectoparasite ''Paulsenella'' and of ''Amyloodinium''. It usually reproduces using asexual fission but is known to exhibit a sexual life cycle as well. Cellular division takes place in non-motile cells, like cysts, that undergo regular mitosis as a diploid zygote. The zygote then undergoes meiosis which results in 2 to 8 bi-flagellated offspring | ''P. shumwayae'' shares phylogenic relationships with and shows similar reproductive patterns to the marine diatom ectoparasite ''Paulsenella'' and of ''Amyloodinium''. It usually reproduces using asexual fission but is known to exhibit a sexual life cycle as well. Cellular division takes place in non-motile cells, like cysts, that undergo regular mitosis as a diploid zygote. The zygote then undergoes meiosis which results in 2 to 8 bi-flagellated offspring. <ref name= Parrow2003/> | ||
In response to unfavorable stimuli, P. shumwayae cells will feed and then become non-motile primary cysts. The size of the cyst depends on how much food material was ingested | In response to unfavorable stimuli, ''P. shumwayae'' cells will feed and then become non-motile primary cysts. The size of the cyst depends on how much food material was ingested. When isolated culture populations were starved, cells resorted to cannibalism. Due to lack of sufficient nutrition, older cysts tend to be smaller in size. <ref name=Parrow2003/> | ||
===Asexual=== | ===Asexual=== | ||
{{Image|P_shum_figure.JPG|right|450px|Diagram of the most commonly observed Pfiesteria shumwayae reproductive cyst development (nucleus represented by black oval)}} | {{Image|P_shum_figure.JPG|right|450px|Diagram of the most commonly observed ''Pfiesteria shumwayae'' reproductive cyst development (nucleus represented by black oval)}} | ||
Reproductive cysts were observed to have several protoplast divisions, internal to the plasma membrane which does not undergo division. (see diagram) The most common form of asexual reproduction of ''P. shumwayae'' cysts begins with a primary cyst. This primary cyst undergoes a protoplast division which can either result in two bi-flagellated offspring (A) or become a secondary cyst (B). The number of divisions and of offspring produced depends on the size of the primary cyst. The secondary cyst undergoes another protoplast division internal to the secondary cyst wall, releasing four bi-flagellated offspring (B). If the primary cyst was extremely large, the secondary cyst would undergo another protoplast division forming a tertiary cyst which releases eight bi-flagellated offspring (C). These divisions occur within several hours of each other. The food vacuole (represented by gray oval) present in the primary cyst is not divided amongst the offspring but grows smaller and is inherited by only one offspring | Reproductive cysts were observed to have several protoplast divisions, internal to the plasma membrane which does not undergo division. (see diagram) The most common form of asexual reproduction of ''P. shumwayae'' cysts begins with a primary cyst. This primary cyst undergoes a protoplast division which can either result in two bi-flagellated offspring (A) or become a secondary cyst (B). The number of divisions and of offspring produced depends on the size of the primary cyst. The secondary cyst undergoes another protoplast division internal to the secondary cyst wall, releasing four bi-flagellated offspring (B). If the primary cyst was extremely large, the secondary cyst would undergo another protoplast division forming a tertiary cyst which releases eight bi-flagellated offspring (C). These divisions occur within several hours of each other. The food vacuole (represented by gray oval) present in the primary cyst is not divided amongst the offspring but grows smaller and is inherited by only one offspring. <ref>Parrow, M.W. and Burkholder, J.M. 2003. Reproduction and sexuality in Pfiesteria shumwayae (Dinophyceae). J. Phycol. 39:697-711</ref> It was observed that the offspring egested the food vacuole after excystment. | ||
===Sexual=== | ===Sexual=== | ||
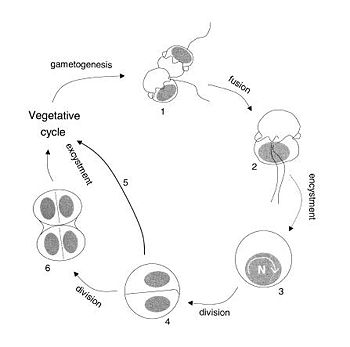
In sexual reproduction of ''P. shumwayae'', two gametes fuse to form a planozygote. The cell engages in nuclear cyclosis which produces two flagellated offspring. Some of the steps in reproduction are unknown because of the difficulty researchers have in documenting information. The vegetative cells and the gametes look very similar, and fusion can easily be confused with division, making it difficult to differentiate between cycle phases | {{Image|sexypfiesteria.jpg|right|350px|Sexuality in ''Pfiesteria shumwayae''}} | ||
In sexual reproduction of ''P. shumwayae'', (see diagram below) two gametes fuse (1) to form a planozygote (2). The cell engages in nuclear cyclosis[http://www.ncsu.edu/wq/harmfulalgae-estuarine/dinoflagellates/pfiesteria/index.html] (3) which produces two flagellated offspring (4). Some of the steps in reproduction are unknown because of the difficulty researchers have in documenting information. The vegetative cells and the gametes look very similar, and fusion can easily be confused with division, making it difficult to differentiate between cycle phases. <ref name=Parrow2003>Parrow, M.W. and Burkholder, J.M. 2003. Reproduction and sexuality in P''fiesteria shumwayae'' (Dinophyceae). J. Phycol. 39:697-711.</ref> That is why after nuclear cyclosis, it is unknown whether another division takes place before the excystment of offspring (5) and (6). According to Parrow and Burkholder’s research, reproduction in ''P. shumwayae'' seems to be unlinked to a survival strategy, and reverts to a sexual cycle because of self- sterility factors that favor outbreeding while taking advantage of food availability and environmental quality. | |||
==Ecology== | ==Ecology== | ||
Fish kills in warm coastal estuarine waters are usually attributed to harmful algal blooms of ''Pfiesteria''. It is not typical for these blooms to occur after hurricanes and storms or in “high-wave-action, wind mixed” surface waters because ''Pfiesteria'' prefers calm, quiet water conditions, like poorly drained brackish water | Fish kills in warm coastal estuarine waters are usually attributed to harmful algal blooms of ''Pfiesteria''. It is not typical for these blooms to occur after hurricanes and storms or in “high-wave-action, wind mixed” surface waters because ''Pfiesteria'' prefers calm, quiet water conditions, like poorly drained brackish water.<ref name=Glasgow2001/> It is implicated that the stress of low oxygen and hypoxia in such waters is the primary cause of these manifestations. | ||
==Current Research== | ==Current Research== | ||
It has been established that ''Pfiesteria shumwayae'' kills fish by feeding on its epidermis. Research by Skelton, Burkholder and Parrow ( | It has been established that ''Pfiesteria shumwayae'' kills fish by feeding on its epidermis. Research by Skelton, Burkholder and Parrow (2008), supports the idea that ''Pfiesteria'' is responsible for fish kills and lesions on wild fish due to myzocytosis and not so much exotoxin release. Many fish kills documented in their on- site field research show that non-inducible and temporarily non-toxic strains of ''Pfiesteria'' were present during kills and were not actively toxic. But Vogelbein et al. (2002) stated that there is some ambiguity of the role ''Pfiesteria'' plays in the mortality of wild fish due to pathogenicity via micro-predation versus exotoxin release. This conclusion is logical because ''in vitro'' assays sometimes cannot discriminate fish kills caused by exotoxins of toxic strains or myzocytosis. It can be suggested that more research be done to propose a definite conclusion to this issue. However, retrieving substantial and accurate information on fish kills using traditional research methods is difficult for several reasons: 1). ''Pfiesteria'' zoospores change phases or life cycle stages, and may settle out of the water column making it harder to detect and quantify 2). Since these occurrences are in the form of blooms, they tend to be ephemeral and 3). There are ''Pfiesteria''–like species present in the field that are morphologically similar to ''Pfiesteria'', making it difficult for researchers to identify and distinguish one from the other.<ref name=Glasgow2001/> | ||
==References== | ==References== | ||
{{reflist}}[[Category:Suggestion Bot Tag]] | |||
Latest revision as of 11:00, 3 October 2024
| Pfiesteria shumwayae | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 | ||||||||||||||
| Scientific classification | ||||||||||||||
|
Description and significance
Pfiesteria shumwayae is a heterotrophic dinoflagellate belonging to the protist group pyrrophycophyta. It was discovered by Dr. Burkholder at North Carolina State University in 1995. These dinoflagellates are found in warm coastal regions and temperate or tropical estuaries, and are known to cause massive fish kills via micropredation. It was once thought that fish kills in these areas were caused by exotoxin secretion by Pfiesteria, however recent research has shown that the parasitic feeding behavior of P. shumwayae is responsible for denuding fish of their epidermis and causing characteristic lesions on dead fish.[1] They also prey on other protists, shellfish tissue, and micro-algal species such as cryptomonads[1]. P. shumwayae cysts and excysted offspring feed on the epidermis of live fish and free floating cells that detach from fish. Pfiesteria is known to exhibit both sexual and asexual reproduction, but asexual reproduction is most common among these dinoflagellates. [2]
It is also known that P. shumwayae can have a serious, negative effect on human health. Fisherman who are frequently exposed to Pfiesteria reported having respiratory irritation, rashes, sores and lesions on their skin. Researchers working with P. shumwayae have noted experiencing headaches, dizziness and short-term memory loss. However, there is no evidence supporting illness in individuals who consumed Pfiesteria exposed seafood [3]
Genome structure
Not much research has been done to sequence the Pfiesteria genome in spite of the fact that researchers are mainly concerned with its harmful environmental effects. Much focus has been put on the actual mechanisms that attribute to fish kills and how to mitigate P. shumwayae damage to fish populations.
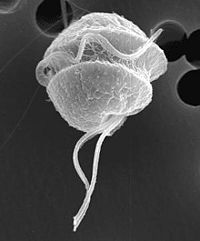
Cell structure and Feeding Habits
P. shumwayae is a biflagellate phagotroph that feeds with a peduncle, or feeding tube which extends from its spherical body. The transverse flagellum is wrapped around it and the other is free flowing. The peduncle is used to ingest epidermal cytoplasm and other food matter via myzocytosis.[1] Direct dinospore attachment to the epidermal surface of fish create abrasions and damage to fish tissue such as gills, olfactory organs, oral mucosa and the lateral line canal. After completion of dinospore feeding, the peduncle is stretched thin and retracts as it detaches from the fish to swim away. [2]
Vogelbein et al. (2002), showed that cell organelles such as mitochondria and rough endoplasmic reticulum was observed as it passed through the peduncle during feeding. As fish cytoplasm is taken up by the peduncle, the dinospore increases in size because the ingested materials are transported to a food vacuole that occupies most of the cell. Staining of the dinoflagellates after feeding revealed that the food vacuoles within them were filled with nucleic acids and lipids. [4]
As P. shumwayae feeds, it uses its peduncle to pierce the cell membrane of its host, allowing host cell cytoplasm to flow through a small circular opening which contracts.[4] This sphincter, observed by Calado and Moestrup (1997), contracts in order to control the amount of cytoplasm being ingested into and pushed out of the dinoflagellate’s food vacuole. Calado and Moestrup suggested a theory of two different mechanisms in which suction serves as the driving force behind cytoplasm ingestion.
During feeding, P.shumwayae exhibits cytoplasmic extensions or pseudopodia that are constantly extended and retracted for movement and attachment to host (Lom and Lawler, 1973). Up to six extensions were observed per cell. As the cell moves in response to chemosensory attraction, cytoplasmic extensions are left behind. Other flagellate cells are attracted by these entrails and were observed as they attached themselves to the entrails and ingested them. [4] After feeding on micro-algae and other prey, P. shumwayae leaves behind only the cell membrane; however it is noted that when it feeds on fish cells under axenic[2] laboratory conditions, no visible cell membrane is left behind. [4] In addition, research documented by Schnepf and Deichgraber (1984) supports that the cell membrane is not ingested during or after P. shumwayae feeding.
It has been noted that P. shumwayae can feed on prey several times larger than its own size; however feeding occurs on organisms that are usually injured ciliates, rotifers and nematodes. It has also been reported that the dinoflagellates can ingest heterotrophic bacteria via phagocytosis, but this is an area that is in need of more research. [4]
Toxicity
The Pfiesteria species contains P. shumwayae and P. piscicida, both of which have benign and toxic strains. The classification of toxicity in strains of Pfiesteria has to do with whether or not their exotoxins are inducible. In benign strains (NON-IND), toxins are not inducible; dinoflagellates are either not capable of toxic activity or the toxins they produce are negligible. Toxic strains that are actively toxic (TOX-A) or temporarily toxic (TOX-B) depend on whether there are live fish present.[5] In the presence of live fish, P. shumwayae exhibits immediate chemotaxis to fish and gradually becomes toxic. According to research done by Glasgow et al. (2001), it was demonstrated that shortly after fish death, TOX-A strains of Pfiesteria ceased production of toxins and transformed into an amoeboid form that fed on dead fish remains. In the presence of other micro-algal prey, P. shumwayae reverted to the less toxic TOX-B form. Optimal temperature for toxicity is greater than or equal to 25°C.
Experiments were done that indicate toxic strains of P. shumwayae use exotoxins to weaken fish. They can also have effects on mammals as well. Toxins secreted by dinospores are similar to an ATP transmitter that targets the P2X7 receptor [5], causing a severe inflammatory response. Cultured fish exposed to Pfiesteria toxins showed signs of depression, loss of equilibrium, hyper-excitability and decreased respiration. The physical, epidermal damage done to fish by Pfiesteria is speculated to increase the efficiency of toxins into fish tissues. Some strains of Pfiesteria that are less toxic do not kill fish unless it has direct contact with its prey.[5]
Reproduction
P. shumwayae shares phylogenic relationships with and shows similar reproductive patterns to the marine diatom ectoparasite Paulsenella and of Amyloodinium. It usually reproduces using asexual fission but is known to exhibit a sexual life cycle as well. Cellular division takes place in non-motile cells, like cysts, that undergo regular mitosis as a diploid zygote. The zygote then undergoes meiosis which results in 2 to 8 bi-flagellated offspring. [2]
In response to unfavorable stimuli, P. shumwayae cells will feed and then become non-motile primary cysts. The size of the cyst depends on how much food material was ingested. When isolated culture populations were starved, cells resorted to cannibalism. Due to lack of sufficient nutrition, older cysts tend to be smaller in size. [2]
Asexual
Reproductive cysts were observed to have several protoplast divisions, internal to the plasma membrane which does not undergo division. (see diagram) The most common form of asexual reproduction of P. shumwayae cysts begins with a primary cyst. This primary cyst undergoes a protoplast division which can either result in two bi-flagellated offspring (A) or become a secondary cyst (B). The number of divisions and of offspring produced depends on the size of the primary cyst. The secondary cyst undergoes another protoplast division internal to the secondary cyst wall, releasing four bi-flagellated offspring (B). If the primary cyst was extremely large, the secondary cyst would undergo another protoplast division forming a tertiary cyst which releases eight bi-flagellated offspring (C). These divisions occur within several hours of each other. The food vacuole (represented by gray oval) present in the primary cyst is not divided amongst the offspring but grows smaller and is inherited by only one offspring. [6] It was observed that the offspring egested the food vacuole after excystment.
Sexual
In sexual reproduction of P. shumwayae, (see diagram below) two gametes fuse (1) to form a planozygote (2). The cell engages in nuclear cyclosis[3] (3) which produces two flagellated offspring (4). Some of the steps in reproduction are unknown because of the difficulty researchers have in documenting information. The vegetative cells and the gametes look very similar, and fusion can easily be confused with division, making it difficult to differentiate between cycle phases. [2] That is why after nuclear cyclosis, it is unknown whether another division takes place before the excystment of offspring (5) and (6). According to Parrow and Burkholder’s research, reproduction in P. shumwayae seems to be unlinked to a survival strategy, and reverts to a sexual cycle because of self- sterility factors that favor outbreeding while taking advantage of food availability and environmental quality.
Ecology
Fish kills in warm coastal estuarine waters are usually attributed to harmful algal blooms of Pfiesteria. It is not typical for these blooms to occur after hurricanes and storms or in “high-wave-action, wind mixed” surface waters because Pfiesteria prefers calm, quiet water conditions, like poorly drained brackish water.[5] It is implicated that the stress of low oxygen and hypoxia in such waters is the primary cause of these manifestations.
Current Research
It has been established that Pfiesteria shumwayae kills fish by feeding on its epidermis. Research by Skelton, Burkholder and Parrow (2008), supports the idea that Pfiesteria is responsible for fish kills and lesions on wild fish due to myzocytosis and not so much exotoxin release. Many fish kills documented in their on- site field research show that non-inducible and temporarily non-toxic strains of Pfiesteria were present during kills and were not actively toxic. But Vogelbein et al. (2002) stated that there is some ambiguity of the role Pfiesteria plays in the mortality of wild fish due to pathogenicity via micro-predation versus exotoxin release. This conclusion is logical because in vitro assays sometimes cannot discriminate fish kills caused by exotoxins of toxic strains or myzocytosis. It can be suggested that more research be done to propose a definite conclusion to this issue. However, retrieving substantial and accurate information on fish kills using traditional research methods is difficult for several reasons: 1). Pfiesteria zoospores change phases or life cycle stages, and may settle out of the water column making it harder to detect and quantify 2). Since these occurrences are in the form of blooms, they tend to be ephemeral and 3). There are Pfiesteria–like species present in the field that are morphologically similar to Pfiesteria, making it difficult for researchers to identify and distinguish one from the other.[5]
References
- ↑ 1.0 1.1 Vogelbein, W.K.; V.J. Lovko & J.D. Shields et al. (1984), "Pfiesteria shumwayae kills fish by micropredation not exotoxin secretion", Biotechnol. Bioeng. Symp 14: 563–571. Retrieved on 2009-04-24
- ↑ 2.0 2.1 2.2 2.3 2.4 Parrow, M.W. and Burkholder, J.M. 2003. Reproduction and sexuality in Pfiesteria shumwayae (Dinophyceae). J. Phycol. 39:697-711.
- ↑ North Carolina Public Health. "Pfiesteria." 2006. North Carolina Department of Health and Public Services. 23 Apr. 2008. <http://www.epi.state.nc.us/epi/oee/pfie.html
- ↑ 4.0 4.1 4.2 4.3 4.4 Skelton, H.M., Burkholder, J.M. and Parrow, M.W. 2008. Axenic Cultivation of the Heterotrophic Dinoflagellate Pfiesteria shumwayae and Observations on Feeding Behavior. J. Phycol. 44:1614-1624
- ↑ 5.0 5.1 5.2 5.3 5.4 Glasgow, H.B.; J.A.M. Burkholder & M.A. Mallin et al. (2001), "… toxic Pfiesteria complex species and a conservative analysis of their role in estuarine fish kills", Environmental Health Perspectives: 715–730. Retrieved on 2009-04-24
- ↑ Parrow, M.W. and Burkholder, J.M. 2003. Reproduction and sexuality in Pfiesteria shumwayae (Dinophyceae). J. Phycol. 39:697-711